Abstract
Introduction: The EMA/FDA-approved, erythroid maturation agent, luspatercept, has been shown to decrease transfusion burden in patients (pts) with transfusion-dependent thalassemia (TDT). Data on efficacy and safety for non-selected pts, not involved in clinical trials, are lacking.
Aim: In this multicenter, retrospective cross-sectional study, real-world data from the use of luspatercept in TDT pts, managed in 9 major Thalassemia Centers in Greece are presented.
Methods: Inclusion criteria included: TDT pts, having received luspatercept as per approved indications, treatment duration for at least 12 weeks. Data cut-off date was 30/06/2022. We estimated the quantity of blood (in cc of Packed Red Blood Cells -PRBC) received over 12 weeks. The following intervals were assessed post starting therapy: 1-12 weeks; 5-16 weeks; 13-24; the last 12 weeks of the observation period and 12 weeks post dose increase. The 12 weeks before starting treatment was considered as baseline. Changes in mean pre-transfusion hemoglobin (Hb), and in biochemical and other hematological parameters, were analyzed. Adverse events (AE) were recorded. Statistical analysis performed with RStudio v.3.6.2.
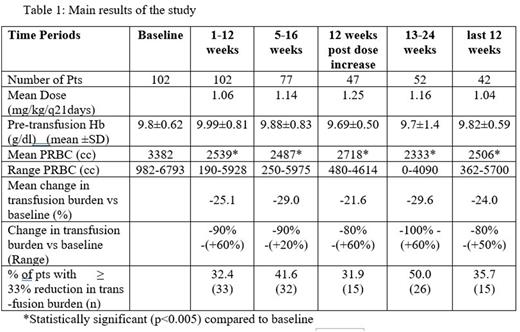
Results: One hundred two pts (median age: 47 years range:25-67, sex: M:F/57:45) received Luspatercept every 21 days. Sixty-nine (67%) pts had transfusion burden >20 PRBC units/24 weeks, which was an exclusion criterion for the registration trial 'Believe'. Pts had variable genotypes, which included: β0/β0 (12 pts), β+/β0 (28 pts), β+/β+ (26 pts), β+/β++(11 pts), β++/β0 (10 pts), β++/β++ (1 pt), not available (14 pts). Luspatercept was administered for a median of 24 weeks (range: 12-70 weeks). The initial dose of luspatercept was 1 mg/kg and increased selectively up to 1.25 mg/kg based on tolerability and efficacy and according to guidelines. Main results of the study are shown in Table 1. A statistically significant (p<0.005) decrease in transfused PRBCs compared to baseline was observed in all 12-weeks’ intervals. There were no significant differences of transfused PRBCs between the initial interval of 1-12 weeks and neither any subsequent 12 weeks’ intervals nor the interval post dose increase. As dose increase was reserved for poor responders, the latter comparison suggests that higher doses are required in this group of pts to reach satisfactory results. There were no significant changes in mean pre-transfusion Hb compared to baseline. The percentage of pts who had a reduction in the transfusion burden of at least 33% from baseline increased with time and at the interval of 13-24 weeks was 49%. Only one pt (42years old, HBB:c.92+1G>T /HBB:c.*6C>G) became transfusion independent.
A statistically significant (p<0.005) increase in uric acid and LDH was observed for the interval 1-12 weeks in comparison with baseline (mean: 5.8±1.7mg/dl vs 5.1±1.6mg/dl, 343±170mg/dl vs 232±99mg/dl, respectively). Similar difference was observed between the 13-24 weeks interval and baseline for uric acid and LDH and for the 24-36 weeks interval only for uric acid and platelets. (mean: 6.1±1.6 mg/dl vs 5.3±1.6mg/dl, 485x10^9±226x10^9/L vs 404x10^9±234x10^9/L, respectively).
Fifty pts (49%) reported at least one AE. The most common AEs included: bone pain 36 pts (35.2%), fatigue 17 pts (16.7%), headache 9 pts (8.8%) and periorbital edema 7 pts (6,9%). Frequent urination, dizziness, hypertension, hyperuricemia, swelling at injection site, blurry vision, tearing, libido decrease, tachycardia, abdominal flatulence, insomnia, dyspnea were also reported.
Treatment was discontinued in 36 pts (35%) after 19.3±8.1 weeks (range 12-48 weeks). Reasons for discontinuation included: no response (15 pts), adverse events (8 pts), patient's decision (6 pts), no response and adverse events (4 pts), adverse events not related to treatment (3 pts; spondylodesis, osteonecrosis of the hip during COVID19 infection and death due to COVID19 infection, respectively). Compared to the registration trial 'Believe', there were higher percentages of pts decreasing transfusion burden ≥33% but also of therapy discontinuation.
Conclusions: Real world data on the use of luspatercept in TDT parallel results from the registration trial, showing heterogeneous and lasting efficacy and acceptable toxicity, despite including pts with different characteristics. Longer follow up and increased number of pts are required to validate these observations.
Disclosures
Kourakli:Gilead: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; Jenssen: Consultancy, Honoraria; AstraZeneca: Honoraria; Pfizer: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Delicou:GENESIS Pharma SA: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Research Support (PI); DEMO: Honoraria; Genesis Pharma SA: Research Funding; Ionis Pharmaceuticals: Other: Clinical Trial Research Support (PI); Vifor International Inc: Other: Clinical Trial Research Support (PI); Forma Therapeutics Inc: Other: Clinical Trial Research Support (PI). Diamantidis:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; GENESIS Pharma SA: Honoraria, Research Funding; Uni-Pharma: Honoraria; Ionis Pharmaceuticals: Other: Clinical Trial Research Support (PI); NOVARTIS: Other: Clinical Trial Research Support (PI); Vifor International: Other: Clinical Trial Research Support (PI); Forma Therapeutics Inc. and Synteract: Other: Clinical Trial Research Support (PI). Symeonidis:Vianex: Research Funding; Roche: Research Funding; WinMedica: Research Funding; Rafarm: Honoraria; Astellas: Research Funding; Abbvie, Amgen, Astra-Zeneca, BMS, GenesisPharma, Gilead, Glaxo, Integris, Janssen, Novartis, Pfizer, Sanofi, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Demo/Apopharma: Research Funding; Servier SOBI: Membership on an entity's Board of Directors or advisory committees. Xydaki:Ionis Pharmaceuticals: Other: Clinical Trial Research Support (PI); NOVARTIS: Other: Clinical Trial Research Support (PI); Vifor International Inc.: Other: Clinical Trial Research Support (PI); Forma Therapeutics Inc: Other: Clinical Trial Research Support (PI). Kattamis:AGIOS Pharmaceuticals: Consultancy; IONIS: Consultancy; ViFOR: Consultancy; VERTEX: Consultancy, Honoraria; Chiesi: Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; AMGEN: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal